J. E. Rehder (1989) argues that it is impossible to carburize iron to steel in a forge hearth. Experiments by Sven Rinman (1782), and Ole Evenstad's account (1790) of steelmaking by Norwegian smiths, show empirically that this is incorrect. The flaw in Rehder's theoretical argument probably lies in the use of inappropriate figures for the CO-CO2 ratio vs. temperature in the fuel bed.
In a recent article in Archeomaterials J. E. Rehder (1989: 30-32) argues that carburization of iron in a forge hearth is a practical impossibility: "To carburize iron, it must be enclosed with a source of carbon so as to exclude all or nearly all the products of combustion" (p. 27). Stated in this very general form the thesis is clearly incorrect, for steel can be produced in a bloomery, as Rehder himself states (pp. 28, 29; see also Rehder 1986: 88). In a bloomery the iron is fully exposed to the products of combustion.
Furthermore Sven Rinman (1782, pars. 81 & 268, pp. 333-335, 944-945) describes several experiments in which wrought iron was carburized to steel or even cast iron in a small forge hearth. One of these was as follows:
A soft iron bar,inch square, which after heating and quenching in water did not obtain any hardness, was wrought at one end to a point. In charcoal before a blast of air this was allowed to take a good welding heat, strongly sputtering with fine white sparks, without casting any sand on it. At this heat the point was quickly quenched in cold water; whereafter it was found to be completely clean and white. As far as the welding heat had reached it was so hard that no file could bite it. It was especially hard at the point, where it had begun to melt into a little drop. . . . (Rinman 1782, par. 268, p. 944)
The famous description by Ole Evenstad (1790) of traditional Norwegian iron-production techniques includes a clear description of the making of steel in a forge hearth. This can be seen well enough in Niels Jensen's translation (Evenstad 1968: 65), but since that translation is highly abridged and also rather imprecise I have retranslated the relevant passages in Appendix 1 below. The clarity, precision, and detail of Ole Evenstad's account make it virtually certain that he is telling the truth when he claims to be reporting on his own observations and experiments. Most important is the fact that many details can easily be explained on the basis of modern metallurgical knowledge but could not, in the eighteenth century, have been arrived at by abstract reasoning.
Evenstad's Chapter 8 describes a technique by which bloomery iron is consolidated and purified of slag inclusions by melting in the forge hearth. This part of the process has been tested by Robert Thomsen (1975: 31-33) and found to work. Chapter 9 then describes how this purified iron can be carburized to steel in the same hearth. To understand the text it is only necessary to note that Evenstad apparently assumes the use of a hearth with a sand firebed and with a horizontal tuyère blowing from the side. John D. Light's reconstruction (1987: 661) of an eighteenth-century American charcoal-fueled forge hearth would fit the description almost perfectly. The only obvious difference is indicated by Evenstad's instruction to "pile the fuel high over the edge", which implies that his hearth had a somewhat raised edge.
Evenstad's very clear description of this steelmaking process can be very useful when one attempts to make sense of a number of more obscure eighteenth-century descriptions of steelmaking processes. Note in particular Swedenborg 1734: 207-208 (cf. 113-114; 1923: 246, 140);[1] Rinman 1782, pars. 267-268, pp. 939-945; Ali 1826; J. A. Cramer in Smith 1968: 115-117.
These facts: that steel can be produced in a bloomery, that Sven Rinman in his experiments produced both steel and cast iron in a forge hearth, and that iron was routinely carburized in the forge hearth by Norwegian smiths in the eighteenth century, indicate that there is some flaw in Rehder's argument. We must consider what this may be.
The argument relies on two curves. Rehder's Figure 2 (p. 30) gives
"CO-CO2 ratio in combustion gases vs. temperature", and
Figure 3 (p.
31) gives "CO-CO2 ratio to carburize iron vs.
temperature".
Comparison of the two indicates that for all practical purposes
there is no
temperature at which iron can be carburized by combustion gases.
No source is
given for either curve, nor is the precise meaning of "CO-CO2 ratio"
stated. Is it a ratio of weights or of partial pressures or of
something else?
Figure 3 gives much higher CO-CO2 ratios than those
given in
standard textbooks for the ratio of partial pressures with
= 1 atm; see Figure 1 of the present article (from Rosenqvist
1974: 271; note
also Appendix 2 below). For example, at 950deg.C the value of
required for an equilibrium carbon content of 0.5% in austenite is
about 20,[2] while the "CO-CO2 ratio" for the
same conditions in Rehder's Figure 3 seems to be about 120. Since
Rehder does
not state what he means by this ratio it is necessary to assume
that he has
used a reliable source and that the ratio has the same meaning in
Figure 2 as
in Figure 3.
Rehder's Figure 2 clearly does not represent equilibrium states, for it indicates that the CO-CO2 ratio decreases with increasing temperature. The Boudouard reaction, C + CO2 = 2CO, gives at equilibrium a PCO/PCO2 ratio which increases with temperature (see again Figure 1).
It is correct, of course, that the Boudouard reaction will not reach equilibrium in an open fuel bed like a forge hearth, so that some lower ratio than that given in Figure 1 is to be expected. Carburization can, however, take place at CO-CO2 ratios which are considerably below the Boudouard equilibrium values. It is therefore unfortunate that Rehder does not inform us of the conditions under which the "typical" ratios (p. 31) in his Figure 2 were measured. Clearly the exact geometry of the hearth as well as many other factors will influence how closely equilibrium is approached in the fuel bed. In an earlier article Rehder himself (1986: 88) affirms that an extremely high CO-CO2 ratio can be obtained at 1600deg.C in a relatively small open fuel bed. A "typical" hearth geometry is of limited relevance in an impossibility proof, which must provide for all historically possible hearths.
Excerpts from Ole Evenstad's Treatise on the iron ore which is found in the bogs and marshes of Norway, and the method by which it is transformed into iron and steel (1790), translated from the Danish.
[From the introduction, p. 391:]
... The iron ore which is found in mountains and lakes requires a more artful and costly treatment than bog iron. The former demands a well-equipped ironworks, the latter on the contrary only a bloomery furnace, a common forge hearth, and some other almost insignificant implements. It is bog iron, therefore, which the farmer normally should make use of and learn to treat; therefore I have made many experiments with it, and these have rewarded me richly.
A righteous man makes no secret of the knowledge which he discovers and owns which can promote the common good. With this thought the access to knowledge through my experiments has been open to my neighbours; but I should never have ventured to publish on the subject if the invitation of the Agricultural Society had not, as it were, imposed on me the duty of making my knowledge known for the thorough consideration of others. Thus every noble-minded reader will excuse whatever shortcomings should be discovered in this treatise.
[pp. 437-441:]
Iron which is only melted [i.e. smelted] from ore to raw iron in the bloomery, even if it is of the best kind, is not suitable for use; for although it can be forged and worked to coarse implements, when it breaks it cannot be welded together again. It has still some foreign parts in it which it must be separated from before it is perfectly good; this can be done by remelting in the forge.
Many, even experts, when they see bloomery iron of the best sort, have difficulty judging whether it has been remelted or not, and thus great frauds can be perpetrated with such iron; however a very certain distinctive feature is that the iron is blackish and dark when it has only been melted [i.e. smelted] in the bloomery, while on the contrary it is bluish when it has been remelted.
And this remelting is done in the following manner. From the hearth are cleared all impurities, including coal-remnants, in such a way that the cavity is one inch deep under the tuyère. It must not be deeper, for it must be possible for the draught to reach the iron in the cavity, to cool it; otherwise one obtains steel instead of iron. The cavity is made 11 to 12 inches long from the tuyère side and about 10 inches broad; it is pounded compact, flat, and level at the bottom, so that it does not incline to any side. The bellows and the tuyère must be placed in such a way that the draught goes precisely forward, neither up, down, nor to either side.
The hearth is filled with a pile of coal [i.e. charcoal]; when it is burning well, a half or a whole bloom of iron is laid upon it. As soon as the iron lump is so hot that it casts sparks it is grasped with tongs and held close over the path of the draught, whereafter it melts and falls down in the hearth. While the lump is being held in this way, and is melting, some dry sand, together with finely powdered smithy slag which is completely free from copper or other earth, is cast on the fire. For this purpose the best slag is that which falls from the iron when it is taken up from the bloomery and when it is cloven; this should therefore be collected and kept.
When the lump of iron and the slag have melted and fallen down in the hearth the iron takes the form of a flat lump; the flatter it is, the better the iron. This lump is taken up immediately with tongs; on a large stone, which must be ready in the forge, it is held at the edge and cut into pieces as desired. Then it is ready to be worked up as one wishes.
In the first remelting [of the day] a part of the weight of the iron is often lost, most often when one is not well supplied with a good quantity of good forge slag; but if this is not lacking, and one continues to melt, then little or nothing will be lost. It is therefore advantageous to perform many remeltings at one time, directly following one another. That the slag and other impurities must be cleared from the hearth between remeltings goes without saying.
This remelting, and the forming of the iron to whatever form one wishes, can without doubt be done with less effort and more profit when one has the opportunity to build a little melting-shop which includes a hammer, and in which both the bellows and the hammer can be driven by water. I have made a start at this but have not yet finished it, and thus cannot give a reliable description; especially considering that I have learned nothing of mathematics or mechanics, but must by my own reflection and experimentation find the correct proportions in the arrangement; whence it follows that attempts at various arrangements for this melting- and hammer-shop will perhaps in the beginning be unsuccessful.
When in time I have perfected it I shall with the same willingness acquaint my compatriots therewith, as I have acquainted them with bog iron and its transformation into iron and steel.
When one has used the hearth almost a whole day for the remelting of iron, so that it is well heated throughout, it is cleaned, dug out, and pounded in the same way as for iron-melting; except that it is to be 2 inches deep under the tuyère, so that the draught does not touch the steel when it, upon melting, falls down in the hearth. Then it is filled high over the edge with pine coal. One must then have ready at hand a piece of iron; as much as is remelted at one time, which after remelting must be cloven in pieces and heated and again welded together, and on the anvil hammered out to a square iron bar. This is laid on the coals after they are burning, and when it has been well heated at the one end it is taken with tongs and held in the fire close over the draught from the tuyère; but just high enough that the draught does not touch it. The fire must be strong and sustained, and therefore it is necessary that the draught be constantly strong. With this the iron melts, falls gradually down in the hearth, and becomes steel. Sand is cast on the fire 2 or 3 times during the melting; after it [the iron] is all melted the strong fire is maintained and sand is cast upon it until the steel has settled, so that it can be taken up with the tongs, which is done very carefully, so that it does not go to pieces. As soon as it is taken from the hearth it must be welded and hammered slightly and gently until it is flat; then it is cloven with an axe into small strips and forged out and welded where it is porous, after which it is ready to be worked up. One obtains about half as much steel as the iron that was melted for the purpose.
A bloomery bellows is more suitable in this remelting than the ordinary bellows of a smith; for by the use of the former is melted both more powerfully and in larger quantities at a time than with the latter.
[From the concluding remarks, p. 448:]
. . . Now bloomery production is not exactly new in these parts, but its true advantage, its most advantageous disposition, and its proper treatment have, to my knowledge, been covered in darkness. The timber trade caused bloomery production to be almost entirely forgotten more than 60 years ago; only a few poor peasants in the neighbouring parish, living far in the bush and lacking other occupations, have maintained it until this time, but without great profit because of the lack of both acumen and capital.
...
The curves given here in Figure 1 are strictly correct only
when CO and
CO2 are the only gases present, = 1 atm. Since this is an unrealistic assumption for the
combustion gases in an
open fuel bed, it may be wise to take a step backward to more
basic principles.
For a more detailed explanation of the following see Rosenqvist
1974,
especially chapters 3, 4, and 9. To calculate the relation between
CO and
CO2 at equilibrium in the Boudouard reaction we write
the reaction
as the difference of two formation reactions and consider the
standard free
energies of formation as functions of the temperature T (deg.C).
Rosenkvist (1974: 517) gives empirical curves for and
.
These are approximately linear in the temperature T, and
the expressions
given above are as accurate as the data permit.
The relation between CO and CO2 at equilibrium is then given by the reaction isochore:
(1)
Here R = 1.987 . 10-3 kcal/(deg.K . mole) is
the gas
constant, and
are the respective partial pressures (atm), and K, a
function of T alone, is the equilibrium constant for the
reaction.
The reaction isochore for the cementation reaction, 2CO = CO2 + C(Fe), is similar:
(2)
where the equilibrium constant K is the same as in (1). The activity aC of carbon in austenite is 0 when the carbon content is zero and 1 when the carbon content is the maximum for austenite at the given temperature.
Since 0 < aC < 1 it can be seen immediately by inspection of (1) and (2) that at Boudouard equilibrium the CO content of the combustion gases is always greater than that necessary for cementation.
A very rough approximation for aC at a given weight percentage W of carbon in austenite and temperature 723deg.C < T < 1153deg.C is
(3)
This is derived using three approximations: it is assumed that Henry's Law is correct, that weight percentage is proportional to atomic percentage, and that the maximum carbon content of austenite is a linear function of temperature (cf. Rosenqvist 1974: 111, no. 4.8a).
If one wishes to use (1) or (2) to calculate the more convenient
ratio it is necessary to supply a second equation relating
and
.
In a modern cementation process, using a closed cementation box
and an
accelerator (source of CO2) such as BaCO3, a
suitable
relation is
(4)
Combining (2) and (4) gives, after some straightforward manipulation,
(5)
(To obtain the equilibrium conditions for the Boudouard reaction
it is only
necessary to let aC=1 in this equation.) It can
easily be
calculated that (3) and (5) together give approximately the same
values of as Figure 1; the approximation is closest at the highest and
lowest values of
the carbon content W.
For the combustion gases in a forge hearth the appropriate relation is
(6)
where A ~ 0.21 atm is the partial pressure pO2 of oxygen in atmospheric air. (The derivation of (6) takes account of the fact that the volume of the combustion products is greater than the volume of the blast, because two CO molecules correspond to a single O2 molecule.) Combining (2) and (6) gives
(7)
Equations (5) and (7) are not easy to deal with analytically, but
straightforward calculating drudgery gives the result that the
value of given by (7) is always approximately
times that given by (5). More precisely, for 0 < aC <=
1 and 750deg.C <= T <= 1500deg.C the result given by
(7) is always
between 2.18 and 2.88 times the result given by (5). This means
that the
relevant logarithmic curves in Figure 1 all move approximately
0.40 units
downward without a significant change in their relative positions.
This
obviously has no effect on the present question.
Ali, Oostad Muhammed
(ca. 1826) "Persian metallurgical processes", The quarterly journal of science, literature and the arts, 8: 160-161.
Evenstad, Ole
1790 "Afhandling om Jern-Malm, som findes i Myrer og Moradser i Norge, og Omgangsmaaden med at forvandle den til Jern og Staal. Et Priisskrift, som vandt det Kongelige Landhuusholdnings-Selskabs 2den Guldmedaille, i Aaret 1782", Det Kongelige Danske Landhuusholdnings-selskabs Skrifter, D.3: 387-449 + Tab. I-II.
1968 "A treatise on iron ore as found in the bogs and swamps of Norway and the process of turning it into iron and steel", Bulletin of the Historical Metallurgy Group, 2.2: 61-65. Abridged translation of Evenstad 1790 by Niels L. Jensen.
Light, John D.
1987 "Blacksmithing technology and forge construction", Technology and culture, 28.3: 658-665.
Rehder, J. E.
1986 "Primitive furnaces and the development of metallurgy", Journal of the Historical Metallurgy Society, 20.2: 87-92.
1989 "Ancient carburization of iron to steel", Archeomaterials, 3.1: 27-37.
Rinman, Sven
1782 Försök til järnets historia, med tillämpning för slögder och handtwerk. 2 vols., Stockholm: Petter Hesselberg. There is a German translation, which I have not seen: Die Geschichte des Eisens, mit Anwendung für Künstler und Handwerker, tr. by C. J. B. Karsten. 2 vols., Liegnitz: Triepel& Kuhlmey, 1814-15.
Rosenqvist, Terkel
1974 Principles of extractive metallurgy. New York, etc.: McGraw-Hill.
Smith, Cyril Stanley
1968 (ed.) Sources for the history of the science of steel 1532-1786. Cambridge, Mass. & London: Society for the History of Technology & M.I.T. Press.
Swedenborg, Emanuel
1734 Regnum subterraneum sive minerale. [Vol. 2:] De ferro, deque modis liquationum ferri per Europam passim in usum receptis ... Dresdæ et Lipsiæ: sumptibus Friderici Hekelii.
1762 Traité du fer, par M. Swedemborg; trad. du Latin par M. Bouchu. (Description des arts et métiers: Art des forges et fourneaux à fer, par M. le Marquis de Courtivron et par M. Bouchu; 4ème section). [Paris: Guerin & Delatour]. [Repr. Genève: Slatkine Reprints, 1984]. Partial French tr. of Swedenborg 1734.
1923 Mineralriket: Om järnet och de i Europa vanligast vedertagna järnframställningssätten ... Stockholm: Wahlström & Widstrand. Swedish tr. of Swedenborg 1734 by Hjalmar Sjögren.
Thomsen, Robert
1975 Et meget mærkeligt metal: En beretning fra jernets barndom. Varde, Denmark: Varde Staalværk.
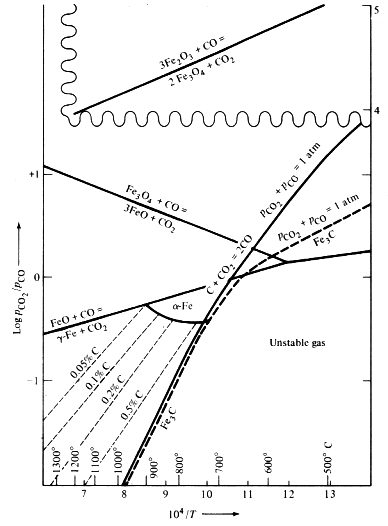
Figure 1. Equilibrium ratio for the reduction of iron oxides and for the Boudouard reaction C
+
CO2 = 2CO. Metastable equilibria for the formation of
Fe3C as well as equilibrium carbon contents in
austenite are given
by dashed lines. Reproduced from Rosenqvist 1974: 271.
[2]See Figure 1: log ~ -1.3, therefore
~ 20.